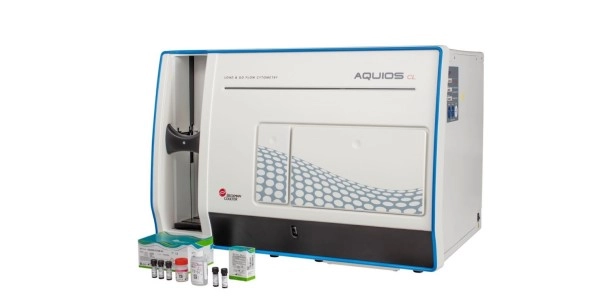
The AQUIOS STEM System, which was developed by Beckman Coulter Life Sciences, have been approved by the FDA. The machine, which was introduced regionally in 2022, revolutionizes stem cell analysis by enabling full automation. It drastically cuts hands-on time by 95%, reduces error-prone procedures by 87.5%, and decreases turnaround time. This system, designed in collaboration with leading experts in the field, employs a modular method to automate the examination of CD34+ hematopoietic stem and progenitor cells for in vitro diagnostics. The new device could benefit blood cancer patients who rely on stem cell transplantation for their treatment of Leukemia. Blood cancers constitute nearly 10% of annual cancer diagnoses in the United States.
According to Dr. Andreas Böhmler, Director of Strategic Marketing for Clinical Solutions, time is incredibly crucial for patients awaiting stem cell transplantation, so they uniquely understood the need to streamline and automate the complex workflow. The workflow has remained relatively unchanged for nearly 25 years, and the AQUIOS STEM System is designed to improve efficiency and enable laboratory staff to accelerate their critical work in analyzing and enumerating viable cells to help improve transplant outcomes.
The AQUIOS STEM System relies on the AQUIOS CL Flow Cytometer, marking the first true load-and-go flow cytometer that combines sample preparation and analysis in one compact platform. Barcoded vials ensure full traceability, providing a comprehensive audit trail. With a single tube loader prioritizing emergency samples, lab staff gain the ability to address urgent requests efficiently.
In a standard 5-day work week handling 10 specimen samples daily, the AQUIOS STEM System reduces manual workload by approximately 6 hours. Moreover, the device reduces the overall turnaround time from sample preparation to patient result, which is crucial for time-critical tests like CD34+ enumeration.
Hans Veenstra, Senior Lab Technician at Radboud University Medical Center's Laboratory for Hematology, highlighted that the an automated stem cell analysis system simplifies the work and saves time. While the AQUIOS CL processes samples, they can focus on other work like complex leukemia and lymphoma analyses. Since the AQUIOS CL does all the sample gating, they only have to check if all the gates are good. It streamlines their daily routine, enabling them to conduct more tests simultaneously.
The AQUIOS STEM System is comprise of the AQUIOS STEM Software for the AQUIOS CL Flow Cytometry System, AQUIOS STEM-Kit Reagents, AQUIOS STEM CD34 Control Cells, and Flow-Check Fluorospheres. The AQUIOS STEM System stands as an in vitro diagnostic (IVD) medical device designed for laboratory professionals for the detection of parameters in commonly used specimen types.